Gene-Edited Fungus Protein: Gene-Edited Fungus ProteinImagine biting into a juicy burger that didn’t come from a cow — but from a fungus grown in a lab. Sounds wild, right? Well, that’s exactly what’s cooking in the world of food science. Thanks to gene-edited fungus protein, we might just be looking at the future of meat — one that’s cheaper, faster, planet-friendly, and surprisingly delicious. Using cutting-edge tools like CRISPR, scientists have developed a new version of a natural fungus that grows quickly, uses fewer resources, and delivers high-quality protein that mimics the texture of meat. This innovation has the potential to transform the food industry, lower environmental impact, and meet global demand for protein — all at once.
Table of Contents
Gene-Edited Fungus Protein
Gene-edited fungus protein may be one of the most promising solutions to the global protein puzzle. With 88% faster growth, significantly lower emissions, and no animals harmed, it combines biotech innovation with real-world practicality. While still under development, the science is sound, the environmental case is clear, and the potential is massive. As regulations shift and production scales up, you might soon find yourself biting into a “burger” that came from a bioreactor — and loving every bite of it.

| Feature | Details |
|---|---|
| What’s New? | Scientists used CRISPR to edit Fusarium venenatum, a fungus used in meat alternatives like Quorn |
| Efficiency Gains | 88% faster protein production, 44% less sugar required |
| Environmental Impact | Uses 70% less land than chicken, with 60% lower emissions |
| Digestibility | Gene edits reduce thick cell walls, making protein easier to digest |
| Regulatory Advantage | No foreign DNA used, simplifying approval processes |
| Applications | Suitable for school meals, military rations, food deserts, and space missions |
| Source | Trends in Biotechnology – Cell Press |
The Global Protein Problem
Let’s set the table with some context. Global demand for protein is skyrocketing, expected to nearly double by 2050 according to the World Resources Institute. Traditional livestock farming — while effective — carries major downsides:
- Livestock contributes about 14.5% of total greenhouse gas emissions, according to the Food and Agriculture Organization.
- It requires vast land, water, and feed resources.
- It’s susceptible to disease outbreaks, zoonotic infections, and supply chain disruptions.
Meanwhile, conventional plant-based proteins have faced pushback over flavor, texture, and perceived unnatural ingredients. Cultivated meats are exciting, but they’re still too expensive and resource-intensive for mainstream production.
What if we could make high-quality protein without the cow, chicken, or pig — faster and cleaner?
Enter: Gene-Edited Fungus Protein
The new solution? A humble fungus known as Fusarium venenatum. This microfungus is already used in making mycoprotein, a type of single-cell protein featured in products like Quorn. It naturally forms fibrous strands similar to muscle tissue, making it an ideal base for meat alternatives.
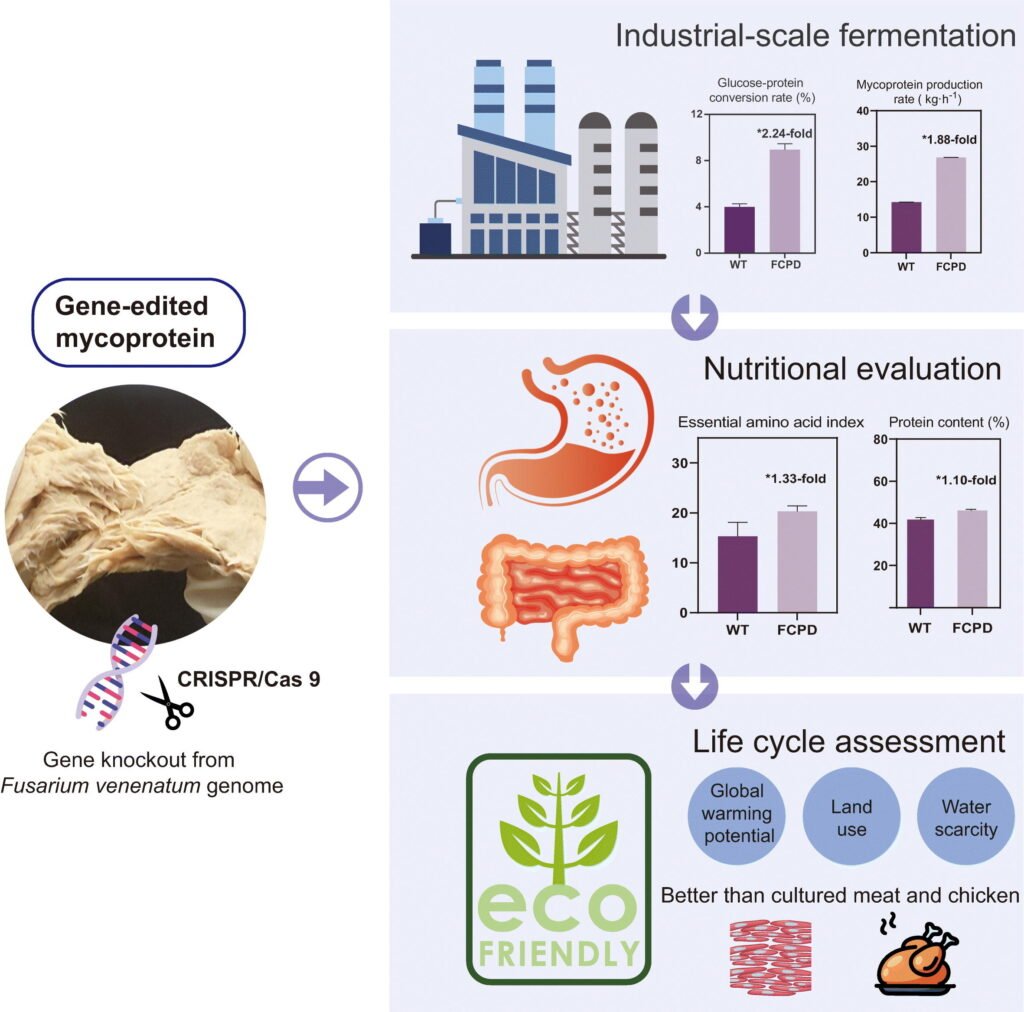
But now, researchers from Jiangnan University have taken it several steps further. Using CRISPR-Cas9, they edited the fungus to enhance its efficiency and digestibility — resulting in a new strain called FCPD.
What’s Changed in the FCPD Strain?
- Chitin Synthase Gene Deleted
This gene is responsible for the tough, fibrous cell walls in fungi. Removing it makes the protein easier to digest, especially for humans who struggle with digesting chitin. - Pyruvate Decarboxylase Gene Removed
This tweak increases how efficiently the fungus uses sugars, allowing it to grow with less input and waste.
Combined, these changes result in:
- 88% faster protein production
- 44% less sugar required for growth
- Thinner cell walls = improved protein absorption by the human body
- Higher protein yield per batch
All without introducing any foreign DNA — just edits to what’s already there.
How Gene-Edited Fungus Protein Works: From Spore to Protein
To better understand why this matters, here’s a breakdown of the production process:
Step 1: Genetic Optimization
CRISPR is used to snip unwanted genes and fine-tune the fungus’s natural processes for optimal growth and efficiency.
Step 2: Fermentation
The fungus is cultivated in large fermenters or bioreactors — similar to how beer or yogurt is made. It’s fed a simple diet of sugar, minerals, and water.
Step 3: Protein Harvest
After a few days, the dense fungal biomass is collected, filtered, and heat-treated to remove excess nucleic acids.
Step 4: Flavor & Texture Design
To make it taste like chicken, beef, or pork, the fungal protein is blended with natural flavors, oils, and seasonings. The texture can be controlled to mimic shredded meat, patties, or nuggets.
Step 5: Pack & Distribute
The final product is shelf-stable or frozen, and can be shipped globally — even to regions that struggle to raise livestock or access fresh meat.
Real-World Use Cases
Gene-edited fungal protein isn’t just lab magic — it has real-world applications with major upside:
- School Lunches: Affordable, nutritious, and allergy-friendly protein for children.
- Military and Disaster Relief: Long shelf-life and lightweight; ideal for emergency rations.
- Space Missions: NASA is already researching fungi as sustainable protein sources for long-term space travel.
- Urban Food Deserts: Small-scale fermenters could allow local production in communities with limited access to fresh food.
- Restaurants and Retail: Food chains could serve meat alternatives that actually taste and feel like meat — without the cost or controversy of animal farming.
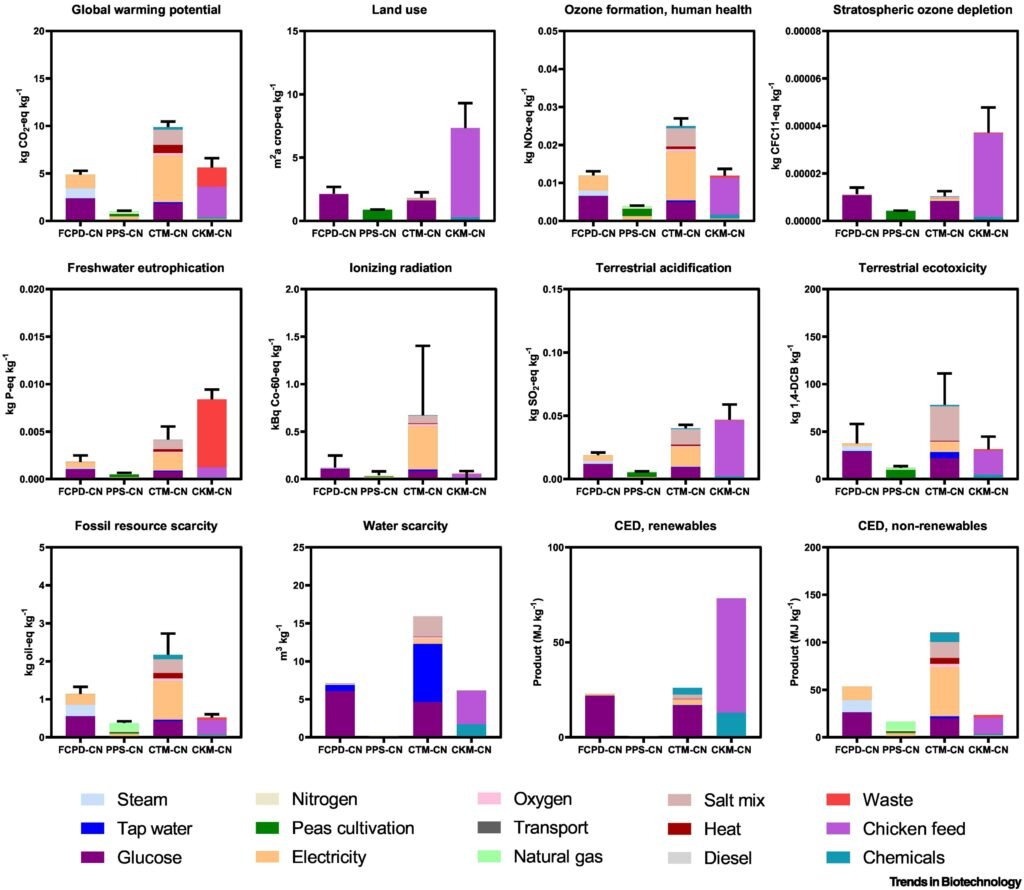
Environmental Impact: Cleaner Protein for a Crowded Planet
Here’s how gene-edited fungal protein stacks up to traditional meat and even other meat alternatives:
| Metric | Chicken | Fungal Protein (FCPD) |
|---|---|---|
| Land Use | High | 70% lower |
| Greenhouse Gas Emissions | Medium | 60% lower |
| Protein Yield (per kg input) | Moderate | High |
| Water Usage | High | 78% lower |
| Growth Time | Weeks | Days |
| Disease Risk | High | Low (sterile environment) |
(Source: Trends in Biotechnology and Nature Sustainability)
These numbers don’t just look good on paper — they offer a real shot at meeting climate targets and food security goals.
Is It Safe and Approved?
Unlike genetically modified organisms (GMOs) that insert foreign DNA, CRISPR gene editing only tweaks or deletes existing genes. This makes it easier to regulate, especially in countries like the U.S., where the FDA and USDA have already shown support for non-transgenic gene-edited foods.
- FDA: Previously approved fungal-based proteins like mycoprotein for human consumption.
- EU: Historically cautious, but beginning to reassess gene-editing under newer frameworks.
- Singapore: First to approve cultivated chicken in 2020, now exploring fungal protein regulation.
Because FCPD doesn’t involve transgenic alterations, experts believe it has a smoother regulatory path, particularly if safety studies confirm no allergens or toxins.
Industry Players and Global Investment
The alternative protein market is booming. According to The Good Food Institute, investments in alternative proteins reached nearly $3 billion in 2022, with a growing portion aimed at fermentation-based solutions.
Key companies innovating in this space include:
- Quorn Foods (UK): Pioneer in commercial mycoprotein.
- Nature’s Fynd (USA): Grows fungus protein from volcanic microbes.
- Meati Foods (USA): Uses mushroom root for meat alternatives.
- Perfect Day: Ferments dairy proteins using fungi (not meat, but relevant tech overlap).
Fungal proteins are considered more scalable and sustainable than cellular agriculture (lab-grown meat), offering a more immediate solution.
Barriers to Adoption
Despite all the advantages, challenges remain:
- Taste Preferences: While texture is spot-on, taste still needs refining to satisfy meat lovers.
- Labeling and Transparency: Terms like “gene-edited” can be confusing or scary to consumers.
- Pricing: Still more expensive than conventional meat, though costs are dropping rapidly.
- Cultural Acceptance: In some regions, fungi are still seen as strange or unappetizing.
Addressing these concerns through education, marketing, and culinary innovation will be critical to success.
Mammoth Cave Fossils Reveal Life From 325 Million Years Ago
Hair Samples Across a Century Show the Real Impact of the Leaded Gasoline Ban
The Legend of the Cursed Amethyst and the Misfortune Linked to It
















