Scientists have unveiled a New Battery Concept that relies on unusual sulfur chemistry to generate electrical power, a development that could expand the frontier of energy storage research. The laboratory-scale design departs from conventional lithium-ion batteries by exploiting sulfur’s ability to undergo complex chemical reactions, potentially allowing significantly higher energy density if technical hurdles can be overcome.
Table of Contents
New Battery Concept
| Key Fact | Detail |
|---|---|
| Core innovation | Uses unconventional sulfur redox chemistry |
| Key advantage | Higher theoretical energy density than lithium-ion |
| Development stage | Early laboratory proof of concept |
| Commercial timeline | Not yet defined |
A Longstanding Search for Better Batteries
Modern life depends heavily on rechargeable batteries, from smartphones and laptops to electric vehicles and renewable energy storage. For more than three decades, lithium-ion batteries have dominated the market because they offer a balance of energy density, reliability, and manufacturability.
Yet lithium-ion technology is nearing its physical limits. Incremental improvements continue, but major gains are becoming harder to achieve. As demand for energy storage accelerates, driven by electric transportation and the transition away from fossil fuels, scientists are increasingly exploring alternative chemistries.
The newly reported New Battery Concept is part of that broader effort. Rather than refining existing lithium-ion designs, it reconsiders how chemical energy can be stored and released at a fundamental level.
Why Sulfur Has Attracted Scientific Interest
Sulfur has long been considered a promising battery material. It is widely available, inexpensive, and capable of storing large amounts of energy relative to its weight. In theory, sulfur-based batteries could outperform lithium-ion cells by a wide margin in terms of energy density.
Traditional lithium-sulfur batteries, however, have faced persistent obstacles. Their chemical reactions tend to produce intermediate sulfur compounds that dissolve into the electrolyte, degrading performance and shortening battery life. This phenomenon, often referred to as the “shuttle effect,” has proven difficult to control.
The New Battery Concept attempts to sidestep some of these issues by embracing sulfur’s complex chemistry rather than suppressing it.
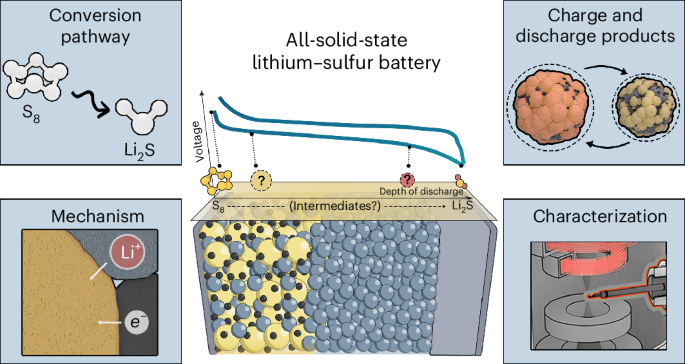
What Makes This Sulfur Chemistry “Unusual”
In most battery systems, the chemistry follows a relatively simple and predictable path. Electrons move through an external circuit while ions shuttle between electrodes inside the cell. The reactions are designed to be reversible and stable.
The New Battery Concept described by researchers takes a different approach. Instead of limiting sulfur to a narrow set of reactions, the design allows sulfur atoms to form intermediate compounds that can transfer more electrons per reaction step.
According to the research team, this expanded reaction pathway increases the amount of electrical energy that can be extracted from a given amount of material. In controlled laboratory conditions, the chemistry demonstrated a higher theoretical energy output than conventional sulfur-based designs.
One researcher involved in the project described it as “using sulfur’s full chemical toolbox rather than forcing it into a simplified role.”
Independent Expert Perspectives
Outside experts caution that promising chemistry does not automatically translate into practical batteries. Many experimental designs perform well in small laboratory cells but fail under real-world conditions.
“The concept is scientifically interesting and well-supported by chemistry,” said a battery materials expert at a U.S. research university who was not involved in the study. “But stability over hundreds of cycles, safety under stress, and manufacturability remain open questions.”
Another expert noted that complex chemistry can introduce new risks. “The more reaction pathways you allow, the harder it becomes to control side reactions that degrade performance or compromise safety,” the expert said.
Such skepticism is common in the field, where bold claims have often faded during later stages of development.
How the New Battery Concept Compares With Other Next-Generation Designs
The New Battery Concept enters a crowded research landscape. Scientists worldwide are exploring alternatives to lithium-ion, including solid-state batteries, sodium-ion systems, and metal-air designs.
Solid-state batteries aim to replace flammable liquid electrolytes with solid materials, improving safety and potentially energy density. Sodium-ion batteries offer lower costs and greater material abundance but generally lower performance.
Sulfur-based systems, including the newly proposed design, stand out for their theoretical energy potential. If their challenges can be solved, they could enable lighter batteries for applications where weight is critical, such as aviation or space exploration.
Still, analysts note that no single technology is likely to replace lithium-ion across all uses. Instead, different chemistries may coexist, optimized for specific applications.
Environmental and Supply-Chain Implications
Beyond performance, battery research increasingly focuses on environmental impact and resource security. Lithium-ion batteries rely on materials such as cobalt and nickel, which are expensive and often sourced from geopolitically sensitive regions.
Sulfur offers a potential advantage. It is widely produced as a byproduct of industrial processes, including oil refining, and is often considered waste. Using sulfur in batteries could reduce reliance on scarce metals and lower overall costs.
However, the environmental profile of the New Battery Concept remains uncertain. Some sulfur compounds involved in the chemistry can be reactive or corrosive, raising questions about safe handling and disposal.
Lifecycle analyses will be required to determine whether the technology delivers net environmental benefits once manufacturing, use, and recycling are considered.
Economic Realities and Commercial Prospects
History suggests that moving from laboratory discovery to commercial battery production is a long and uncertain process. Lithium-ion technology itself took decades to mature and required sustained investment from governments and industry.
The researchers behind the New Battery Concept emphasize that commercialization is not imminent. The work represents an early-stage proof of concept intended to demonstrate what might be possible rather than a ready-to-market product.
Industry analysts say that even under optimistic scenarios, new chemistries typically take 10 to 20 years to reach large-scale deployment. During that time, designs often change significantly as practical constraints emerge.
Safety and Durability Challenges
One of the most critical hurdles for any new battery technology is safety. Batteries must operate reliably under a wide range of temperatures, charging speeds, and mechanical stresses.
The unusual sulfur chemistry used in the New Battery Concept introduces uncertainties in this area. Some of the intermediate compounds formed during operation may be unstable under certain conditions.
Researchers say future work will focus on controlling these reactions more precisely and identifying materials that can contain them safely. Extensive testing will be required before the design can be evaluated for consumer or industrial use.
The Role of Fundamental Research
Despite the uncertainties, scientists say the work highlights the importance of fundamental research in energy storage. Many of today’s commercial technologies originated from exploratory studies that initially seemed impractical.
“Even if this exact design never reaches the market, the insights gained could inform other battery systems,” said an energy storage researcher familiar with the study. “Understanding how to manage complex redox chemistry is valuable in itself.”
Such research is often publicly funded and conducted at universities or national laboratories, reflecting long-term societal interest rather than immediate commercial return.
What Happens Next
The research team plans to refine the New Battery Concept by experimenting with alternative electrolytes, electrode materials, and cell architectures. Independent groups are also expected to attempt replication, a key step in validating the findings.
If the results hold up, the work could influence how scientists think about sulfur chemistry in batteries more broadly. If not, it will still add to the growing body of knowledge guiding future designs.
For now, the concept remains a reminder of both the promise and the difficulty of transforming energy storage at a fundamental level.
Looking Ahead
As governments and industries race to decarbonize transportation and electricity systems, the demand for better batteries continues to grow. Breakthroughs are rare, but incremental advances accumulate over time.
Whether the New Battery Concept becomes a stepping stone or a dead end will depend on years of further research. What is clear is that sulfur, long overlooked and underused, continues to challenge assumptions about how batteries can work.
















